Abstract
Introduction
The prognosis of patients with light chain amyloidosis (AL) depends on the biology of the plasma cell clone and the number and extent of organ involvement (especially cardiac) at baseline. Despite recent improvement in survival outcomes, the 1-year mortality continues to be high. However, the outcomes of patients who survive the initial period after diagnosis are not well described.
Methods
Data of 1,179 consecutive patients with systemic AL seen at Mayo Clinic, within 90 days of diagnosis, between 2006 and 2015, was analyzed retrospectively. Of these, 187 (15.9%) patients were lost to follow-up. The overall survival (OS) was calculated from the date of diagnosis till death from any cause or the date of last follow up. The OS curves were generated using Kaplan-Meier method and the survival differences were estimated using log rank test.
Results
The median age of the patients was 64 years (range; 25.6, 94.5), 65.1% (n=767) were males, 56.6% (n=620) had bone marrow plasma cell (BMPC) >10%, 74.4% (n=849) had cardiac involvement, 16.6% (n=191) had hepatic involvement, 58.7% (n=667) had renal involvement, 58.6% (n=691) had multi-organ involvement and 23.1% (n=239) / 22.2% (n=229) / 25.2% (n=260) / 29.5% (n=305) were in Mayo stages I / II / III / IV. The first line treatment included autologous stem cell transplant (ASCT) in 27.2% (n=289), proteasome inhibitor (PI) based in 20.4% (n=243), alkylator based in 35% (n=372), immunomodulator (IMiD) based in 3.6% (n=38) and steroid based in 1.1% (n=12) of patients, while 10.3% (n=110) did not receive any therapy.
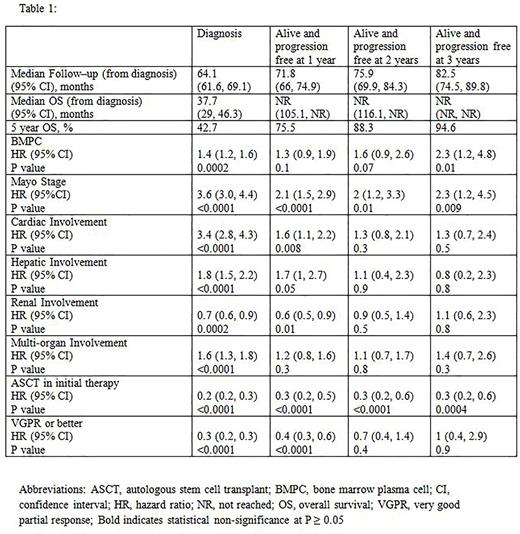
We looked at the impact of following prognostic factors at diagnosis and on follow-up: BMPC (> vs ≤ 10%), Mayo stage (III+IV vs I+II), cardiac involvement (present vs absent), hepatic involvement (present vs absent), renal involvement (present vs absent), multi-organ involvement (>1 vs 1 organ), ASCT in initial therapy (inclusion vs non-inclusion) and very good partial response (VGPR) or better (achieved vs not achieved). The median follow-up, OS, and the impact of various prognostic factors at diagnosis, and for patients who survived without progression after 1, 2 and 3 years are listed in Table 1.
The presence of cardiac involvement, renal involvement and achievement of VGPR or better was no longer associated with OS in those who survived 2 years without progression while hepatic involvement, multi-organ involvement and presence of BMPC > 10% did not have prognostic significance in those who survived 1 year after progression. Advanced Mayo stage and non-inclusion of ASCT in first line treatment predicted worse OS even after being alive and progression-free for 3 years.
Conclusion
Patients with AL who survive the first 1-2 years after diagnosis have excellent long term outcomes. The prognostic value of baseline organ involvement attenuate over follow-up, but characteristics more reflective of the disease biology such as Mayo stage as well as incorporation of ASCT in initial therapy retain prognostic significance long term.
Sidana: Janssen: Honoraria. Dispenzieri: Celgene, Millenium, Pfizer, Janssen: Research Funding. Gertz: Millennium: Consultancy, Honoraria; Celgene, Novartis, Smith-Kline, Prothena, Ionis, Amgen: Honoraria. Dingli: Karyopharm Therapeutics: Research Funding; Janssen: Consultancy; Millenium: Consultancy; Takeda: Consultancy; Alexion Pharmaceuticals: Consultancy. Kapoor: Takeda, Celgene and Amgen: Research Funding. Russell: Imanis Life Sciences: Equity Ownership; Vyriad: Equity Ownership. Kumar: Celgene, Millennium, BMS, Onyx, Janssen, Noxxon, AbbVie, Amgen, Merck, Oncopeptides, Skyline Diagnostics, Takeda: Consultancy; Skyline: Honoraria; Celgene, Millennium/Takeda, Onyx, AbbVie, Janssen, Sanofi, Novartis, Amgen, Genentech, Merck, Oncopeptides, Roche, Skyline Diagnostics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.